Abstract
Introduction: The functional and biochemical characteristics of recombinant proteins are dependent on the amino acid sequence of the protein and the host cell line used to express the protein. We have previously reported the characterization of human cell line-derived recombinant factor VIII (rFVIII) simoctocog alfa (Nuwiq®, Octapharma AG, Switzerland) compared with three other recombinant FVIII products (Advate®, Kogenate FS® and Refacto®) derived from hamster cells and a human plasma-derived (pd) FVIII concentrate (octanate®, Octapharma AG, Switzerland) (Kannicht C et al. Thrombosis Res 2013; 131:78-88; Sandberg H et al. Thrombosis Res 2012; 130:808-17). Here we extend these analyses and compare the characteristics of simoctocog alfa (Nuwiq®) with 2 commercial moroctocog alfa (rFVIII-BDD) products, Octofactor® (Generium, Russian Federation) and ReFacto AF® (Pfizer, USA), to assess potential differences between expression systems and compared with a pdFVIII concentrate (Octanate®).
Materials and methods: Commercially available batches of Octofactor®, ReFacto AF® and Nuwiq® were analyzed. The labeled potencies of all vials were 1000 IU. The reconstitution volumes were 5 mL (Octofactor®), 4 mL (ReFacto AF®), and 2.5 mL (Nuwiq®). FVIII activity was measured using chromogenic, one-stage and FVIII antigen assays. The protein content of the products was analyzed using SDS-PAGE followed by silver staining or Western blotting. Thrombin generation activity (TGA) was measured using Thrombogram at 1 IU. Glycosylation patterns and the extent of sulfation of Tyr1680 were analyzed by the liquid chromatography/mass spectrometry (LC/MS) method. Binding to von Willebrand Factor (VWF) was measured using surface plasmon resonance (SPR) analyses.
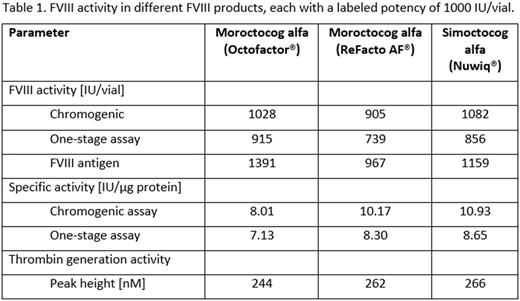
Results: MS analysis confirmed that ReFacto AF® and Octofactor® share the same primary amino acid sequence, including that of the linker sequence between the light and heavy chains. The FVIII activities of each product per vial (labeled potency 1000 IU) are shown in Table 1. The higher FVIII antigen level and the lower specific activity for Octofactor® suggest that these samples contain higher levels of inactive FVIII protein. This is supported by results of the SDS-PAGE analyses, which revealed additional protein bands in the Octofactor® samples that reacted with anti-FVIII antibodies. These bands appear to be partially degraded 170-kDa and 80-kDa protein fragments, suggesting proteolytic cleavage of FVIII during the production of Octofactor®. No significant differences in TGA between the products were detected. The glycosylation patterns of Octofactor® and Refacto AF® were comparable, consistent with both products being expressed in hamster cell lines. Notably, the Octofactor® samples displayed a variable but significantly higher amount of the potentially antigenic non-human Neu5Gc glycan epitope than ReFacto AF®. Consistent with previous findings, no Neu5Gc was found in the samples of Nuwiq®. Nuwiq® is expressed in a human cell line, and it has been shown previously that the oligosaccharide and sialic acid content of Nuwiq® is comparable to pdFVIII.
Conclusions: The Octofactor® samples showed lower specific activity and higher amounts of partially degraded and inactive FVIII protein. Analysis of the glycosylation patterns showed that Octofactor® and Refacto AF® both contain potentially antigenic non-human Neu5Gc glycan epitopes due to their expression in hamster cell lines. These epitopes are not present in Nuwiq®, which is produced in a human cell line, or in octanate®, which is derived from human plasma.
Kohla: Octapharma Biopharmaceuticals GmbH: Employment. Belyanskaya: Octapharma AG: Employment. Tiemeyer: Octapharma Biopharmaceuticals GmbH: Employment. Züchner: Octapharma Biopharmaceuticals GmbH: Employment. Knappik: Octapharma AB: Employment. Rosenlöcher: Octapharma Biopharmaceuticals GmbH: Employment.
Author notes
Asterisk with author names denotes non-ASH members.